KEMH at the forefront of hip replacement surgery
Monday 17 February 2025: Bermuda Hospitals Board (BHB) is part of a US Food and Drug Administration (FDA) clinical study to bring a new orthopaedic implant to the United States. The Polymotion Hip Resurfacing (PHRTM) is a new hip replacement device that will be used in men and women under age 65 as a part of the clinical study. In time, Polymotion may join its predecessor, the Birmingham Hip Resurfacing (BHRTM), as a globally available hip resurfacing device.

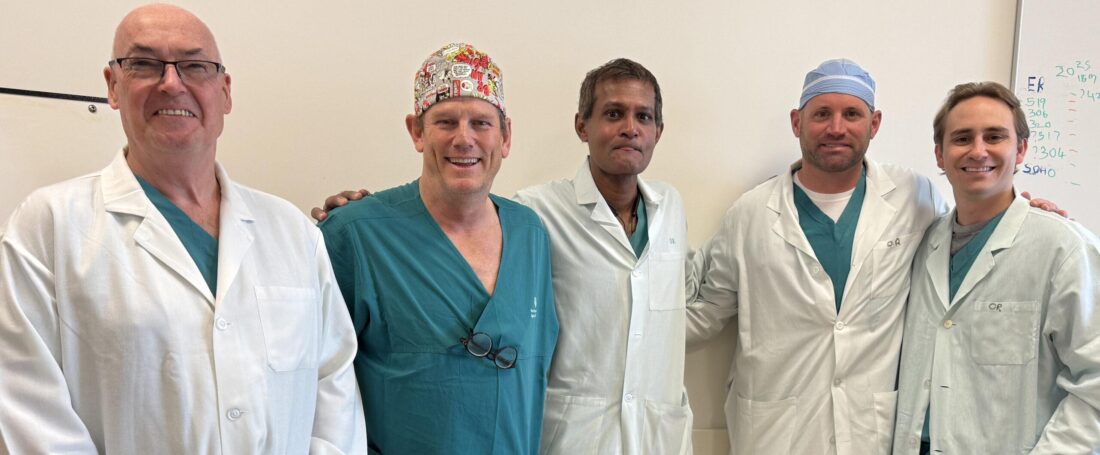
Orthopaedic surgeon Dr Ronan Treacy (pictured left) is a co-inventor of Polymotion. He also designed the Birmingham implant in the late 1990s. He’s been a regular visiting surgeon in Bermuda for over 25 years and has performed hundreds of Birmingham Hip Resurfacing procedures on local residents at King Edward VII Memorial Hospital (KEMH).
BHB Director of Orthopaedics Dr William Peckett has worked with Dr Treacy for over 20 years and has been trained to implant both the Birmingham and the Polymotion devices.
Dr Treacy and Dr Peckett did the first hip replacement surgeries using the Polymotion implant at KEMH in January as part of the Investigational Device Exemption (IDE) study for the FDA.
Dr Treacy said: “I believe we are the first overseas centre ever to have taken part in such a study. We are privileged to be in the company of fantastic centres such as NYU, New York; RUSH University Medical Center in Chicago and some of the biggest names in hip surgery in the world.”
“In total, the team performed the surgery on four patients. Each went home on the day of surgery. All are recovering well.”
Most patients who undergo hip replacement surgery at KEMH are discharged home the same day.
Dr Treacy said: “The patients actually prefer to go home and be in their own bed in their first night. The level of pain control isn’t really an issue. Patients are very mobile afterwards.”
“This is the trend in the United States. We haven’t quite caught up in the UK. In fact, we are far in advance here at the King Edward Hospital to regular practice in the UK.”
Although the Bermuda surgeries were the first in the US study, Dr Treacy has successfully implanted metal-on-polyethylene hip resurfacings (similar to the Polymotion) in Birmingham, UK, for the last 9 years. He has performed about 90 surgeries.
Worldwide, approximately 250 of these metal-on-polyethylene resurfacing devices have been implanted. The Polymotion is currently being used regularly in New Zealand on general release to treat various hip conditions. It is also going through the necessary regulatory processes in other global markets, including Australia and Canada.
Dr Peckett will perform most of the local clinical study cases. He said: “We are excited to be one of only ten centres that were chosen for this study. The other nine are all in the US.”
“Over 200 patients are expected to be enrolled in the study, which will run for at least 2 years. We would be expecting to enroll approximately 20 Bermudian patients in total over that time period.”

JointMedica, manufacturers of Polymotion, were on hand at the surgeries last week. President of the company, Dr. Sharat Kusuma, also an orthopaedic surgeon, said adding a polyethylene lining
to the socket of the metal ball and socket device differentiates it from its predecessor, the Birmingham. Over the past ten years, metal-on-metal hip replacement devices (like the Birmingham) have become less popular due to the metal-on-metal bearing surface. The Polymotion device incorporates several design features of Birmingham resurfacing but eliminates the metal-metal bearing surface.
Dr Treacy said: “Dr Peckett just did his 300th outpatient joint replacement here over the last two years. This is an extraordinary achievement due in large part to the King Edward nursing staff, radiology staff, its anaesthetic staff, and a lot of pre-operative work.”
BHB Chief of Surgery Dr. Alicia Stovell-Washington said: “We are proud that JointMedica has included KEMH as a site for this FDA study; it reinforces that our surgical department has world-class staff and processes.”
We thank Drs Treacy and Peckett for spearheading this effort to improve the lives of our patients.
Bringing the Polymotion clinical study to Bermuda completely aligns with our BHB vision; To pursue excellence through improvement, to make Bermuda proud.”
For more information on the Polymotion device click here.
Pictured at the top, from left: Tim Band, Senior Director Market Development, JointMedica; Dr William Peckett, BHB Director of Orthopaedics; Dr Sharat Kusuma, President, JointMedica; Jason Osier and Warner Watkins, JointMedica distribution agents.
Archives
- 12 January - BHB Traffic Advisory for KEMH campus
- 2 January - Bermuda’s first baby of 2026 is born
- 25 December - Bermuda Hospitals Board welcomes two Christmas babies
- 11 December - Hyperbaric chamber temporary closure
- 22 September - Bermuda Hospitals Board awards $191,000 in scholarships
- 10 September - Dr Michael Ashton takes on new role at BHB
- 8 September - Temporary KEMH Road Closure on Wednesday 10 September
- 17 July - BHB welcomes 45 summer students
- 3 April - Update on bed boarding status at KEMH
- 19 March - BHB moves to Alert Level 2
- 19 March - BHB update on Oncology service
- 12 March - BHB introduces two new oncologists
- 12 March - Paul Jones joins BHB as chief people officer
- 21 February - Public Advisory: Hyperbaric chamber closed temporarily
- 20 February - KEMH through road closed Friday 21 February 2025
- 19 February - Deadline for BHB summer student programme one week away
- 17 February - KEMH at the forefront of hip replacement surgery
- 6 February - KEMH through road closed Friday 7 February afternoon
- 24 January - BHB Public Advisory on Patient Billings
- 23 January - Oncology update
- 27 December - Lamb Foggo Urgent Care Centre closed on Saturday
- 16 December - Celebrating blood donors this Christmas
- 16 December - Stroke and the holidays
- 10 December - BHB urges online bill payment
- 7 November - Sterile processing department at KEMH to be upgraded
- 1 November - Oncology Service Notice
- 22 October - Reduce your stroke risk with physical activity
- 15 October - Caring for patients at KEMH
- 6 October - BHB to host Mental Health Awareness Fair
- 18 August - Baby Sarai born in the eye of Ernesto
- 29 July - BHB awards $190,000 in scholarships
- 18 July - No through traffic at KEMH on 20 July
- 20 June - No through traffic at KEMH on 21 June
- 10 June - No through traffic at KEMH on 12 June
- 30 May - BHB MRI service up and running
- 22 May - BHB’s MRI temporarily down
- 12 March - BHB celebrates World Kidney Day
- 29 February - Generous donation after life-saving cardiac care
- 3 January - Bermuda’s first baby of 2024 is born
- 25 December - Two babies born on Christmas Day
- 18 December - Scott Pearman appointed as BHB CEO from January 2024
- 15 December - Only visit your loved ones in hospital if you feel well
- 29 November - Green is good for your mental wellness
- 22 November - Donation brings new treatment for enlarged prostate
- 17 November - BHB resumes pre-COVID visiting hours
- 10 November - BHB histopathology tests and post-mortem reports
- 29 September - BHB turns on pink lights
- 18 September - BHB to host free Falls Prevention Mini Expo
- 13 July - Blood donor raffle winners announced
- 24 May - Free CPR training could save a life
- 27 February - Faster and more efficient laundry now in operation
- 6 January - KEMH welcomes first babies of 2023
- 3 January - BHB looks forward to PEARL benefits
- 28 December - BHB COVID update
- 25 December - Three babies born on Christmas Day
- 22 December - BHB jobs fair attracts over 300
- 21 December - Community initiative for old St. James’ Church rectory
- 5 December - BHB updates COVID guidelines
- 1 December - O positive blood donors urgently needed
- 30 November - BHB shines green light for mental health awareness
- 17 November - BHB Traffic Advisory for Monday 21 November 2022
- 13 November - PEARL Implementation Update
- 9 November - O positive blood donors needed
- 3 November - Local newborn makes BHB history
- 29 October - PEARL Go-Live Milestone Celebrated
- 27 October - BHB prepares for PEARL’s implementation
- 19 October - Urgent call for blood donations
- 12 October - HAB donates biomedical van
- 10 October - Residents rate their mental health
- 30 September - Pink lights at KEMH for breast cancer awareness
- 27 September - BHB welcomes new chief hospital information officer
- 23 September - KEMH welcomes baby during hurricane force winds
- 20 September - BHB’s electronic medical record to go live in October
- 20 September - BHB Public Advisory: the hyperbaric chamber has reopened
- 6 September - BHB awards $100,000 in scholarships to eight students
- 9 August - Donate blood and win a ride on a Whip
- 24 July - BHB laundry gets $6.6 million upgrade
- 3 February - BHB CT scan service has resumed
- 27 January - BHB and Brown Darrell collaborate on CT service
- 9 December - BHB COVID-19 Remembrance Tree
- 30 November - BHB turns on green light for mental health awareness
- 2 November - BHB to ease visiting restrictions starting Wednesday
- 22 October - BHB moves to Disaster Alert Level 3
- 18 October - Lamb Foggo Urgent Care Centre resumes normal hours
- 28 September - BHB morgue service impacted by the pandemic
- 17 September - BHB Moves to Highest Alert Level
- 7 September - BHB visitor restrictions reintroduced due to delta surge
- 27 July - HSBC brings Cup Match magic to BHB
- 22 July - BHB cautiously opens up to more visitors
- 21 July - BHB announces breast ultrasound service
- 9 July - Blood donor winners announced
- 13 May - BHB releases BE KIND logo
- 30 March - BHB update in response to COVID-19 surge
- 24 February - BHB Food Services team completes nutrition course
- 2 February - UCC returns to longer weekday hours
- 1 February - Preston Swan takes on acting COO role
- 26 January - More visitors allowed from 27 January
- 18 January - Mental Health Act Code of Practice now available
- 18 January - Bermuda Hospitals Board COVID-19 vaccination update
- 15 January - BHB pilots thermal detectors at its entrances
- 8 January - Why I will be getting the COVID-19 vaccine
- 6 January - New year’s baby makes a late appearance
- 29 December - Update on BHB Long Term Care COVID-19 Cases
- 24 December - BHB Confirms Two Patient Cases in Long Term Care Area
- 23 December - Call for new blood donors to help over the holidays
- 17 December - Dr Wesley Miller appointed as BHB chief of staff
- 14 December - Lamb Foggo UCC Winter Weekday Hours
- 13 December - BHB gives mental health the green light
- 27 November - BHB response to 27 November Royal Gazette article
- 27 October - BHB celebrates Stroke Awareness Week
- 5 August - Agape House maintenance In August
- 24 July - Road closure at KEMH on 27 July
- 21 July - Dr Michael Richmond appointed as BHB CEO
- 2 June - BHB moves to limited visitation
- 19 May - A COVID-19 birthday story
- 29 April - Please wear a mask to the hospital
- 15 April - Police, Fire and Regiment salute KEMH staff
- 13 April - KEMH creates more isolation rooms
- 8 April - Ace Barber Unit converted for pandemic
- 20 March - Only essential visiting now allowed at BHB
- 18 February - BHB releases internal management accounts data
- 18 February - BHB road traffic accident statistics for January 2020
- 31 January - Winter hours at Lamb Foggo UCC extended to February
- 6 January - BHB seeks students for 2020 summer programme
- 1 January - BHB welcomes first babies of 2020
- 2 December - BHB awarded top level of accreditation
- 26 September - World Heart Day: BHB offers free CPR training
- 19 September - BHB welcomes Storm
- 16 September - Hurricane Emergency Kit Guide for Diabetes Patients
- 10 September - 2019 Scholarship Winners Announced
- 4 September - BHB CEO Notifies of Early Retirement in 2020
- 14 August - Improved diabetes management for inpatients
- 16 April - BHB celebrates occupational health nurses
- 25 March - BHB implements hand hygiene audit
- 11 March - Public Advisory: KEMH through road closure
- 22 February - KEMH asphalt resurfacing works
- 6 February - Public Advisory: KEMH roof sealing works
- 5 February - Red Cross Donates Scooter to Gordon Ward Residents
- 22 January - BHB Public Advisory on the Flu
- 5 December - Bermuda Blood Donor Centre thanks polo shirt donors
- 23 October - Walk-in Mammography Day on Thursday
- 12 September - Bermuda Hospitals Board offers free hands-only CPR training
- 26 February - Bermuda Hospitals Board now offers Cardiac CT Scanning
- 14 February - A Valentine’s gift for regular blood donors
- 25 December - Bermuda Hospitals Board welcomes Christmas baby 2017
- 24 November - Public Advisory: Lamb Foggo UCC closed this weekend
- 18 October - KEMH Mammography Department puts breast health first
- 16 October - Bermuda Hospitals Board Traffic Advisory
- 19 September - Bermuda Hospitals Board launches island-wide asthma campaign
- 14 September - Bermuda Hospitals Board has two certified dementia trainers
- 14 September - Lamb Foggo Urgent Care Centre closed this weekend
- 23 March - CEO Venetta Symonds on bed capacity at KEMH
- 17 March - Corporate Blood Drive competition heats up
- 22 February - Lamb Foggo UCC to reopen 1 March
- 1 February - Ms Patrice Dill leaves BHB
- 24 January - BHB introduces Patient-Centred Medical Home
- 20 January - Community invited to open up about BHB services
- 2 January - BHB welcomes New Year’s baby
- 26 December - BHB welcomes Christmas baby
- 22 December - Notice: Holiday hours at UCC and Emergency Department
- 3 December - King’s College urologist joins BHB
- 6 November - BHB focuses on ethics and youth
- 10 October - Being Alive
- 28 September - General Wing Lobby Doors Closed on Thursday 29 September
- 27 September - END OF FISCAL YEAR (2015/16) UPDATE
- 16 September - Urgent Care Centre Closure this Weekend
- 7 September - BHB Update on the Lamb Foggo Urgent Care Centre
- 1 September - Outpatient clinics at KEMH have moved
- 5 August - New hours at Craig Appin House reception
- 25 July - Call for O Negative Blood Donations
- 24 July - Agape House has reopened
- 21 July - BHB Releases CEO Salary Data
- 15 July - BHB Releases 2012 Annual Report
- 16 May - BHB releases salary data
- 4 May - Teen suicide public debate
- 27 April - Dr Keith Chiappa Appointed as Chief of Staff
- 22 February - Agape House 25th anniversary
- 10 February - Update on Genito-Urinary Cancer Care
- 8 February - Blood Donor Centre request to recent donors
- 3 February - BHB Rehabilitation Day Hospital temporarily moved
- 3 February - MRI service at BHB has resumed
- 2 February - Agape House now in Curtis Ward
- 12 January - Kiwanis Clubs Donate $21,000 To Maternity
- 6 January - MRI service interruption
- 5 January - BHB to demolish two buildings on KEMH campus
- 30 December - BHB Posts Hospital-Wide Accreditation Report to Website
- 5 October - Hurricane Joaquin babies at KEMH
- 28 September - MWI sewage treatment plant completed
- 14 September - Acute Care Wing first anniversary – shorter hospital stays
- 18 June - Second Oncologist Joins BHB
- 21 May - BHB Spring Talent Showcase
- 15 April - CCU patients have a new home in KEMH
- 16 February - BHB to dispose of condemned items
- 12 February - Bermuda Hospitals 2014 Update
- 5 February - Operating Rooms now in the Maternity Ward
- 14 September - Emergency Department opens in new Acute Care Wing
- 4 September - Acute Care Wing opens next week
- 29 August - Letter to the Editor of The Royal Gazette
- 12 August - Acute Care Wing opens next month
- 22 July - Tour D´oeuvres and Public Tours
- 15 July - Chance to win a tour of the new wing
- 4 July - Consultant Geriatrician Appointed
- 19 June - BHB mount exhibition
- 9 June - Retiring Blood Donors Honoured
- 15 May - The Back-up generators
- 28 April - We´re connected!
- 21 April - Acute Care Wing: – new features on the face
- 16 April - Atrium- Bringing light to the interior
- 10 April - The New Emergency Department
- 13 February - BHB introduces Eddie the Elephant
- 30 January - Road Works on Point Finger Road this weekend
- 27 January - BHB announce revised opening date
- 16 January - Craneiom comes down
- 7 January - New Chief of Pathology Appointed
- 29 November - Urgent Care Centre Hours Confirmed
- 28 November - Dr Sam Mir Appointed Director of Cardiology
- 14 November - Chairman, CEO speak at Sandys Rotary
- 1 November - BHB Goes Back to Ethics Basics
- 31 October - Quarterly BHB Update – October 2013
- 15 October - New Acute Care Wing Construction Update
- 10 September - CEO and Chairman Speak at Hamilton Rotary
- 6 September - Planned Power Outage at MWI this Weekend
- 26 August - New temporary pedestrian entrance to KEMH
- 24 July - BHB Board Update – 24 July 2013
- 30 April - New Chief of Staff Appointed
- 5 February - Auditor General Informs BHB of Review
- 1 November - BHB Tackles Healthcare Ethics in the Digital Age
- 8 October - Mental Health Awareness Week Tackles Depression
- 2 October - Clinical & Corporate Governance Review Update
- 27 September - Voices Against Stigma: MindFrame PhotoVoice 2012 Launches
- 19 September - BHB´s 2011 Annual Report Released
- 7 August - Chief of Human Resources Appointed
- 16 February - BHB Sees Patient Satisfaction with Services Rise
- 16 February - New CT Scanner Provides Faster and More Accurate Results
- 10 February - Road Works Planned for South Shore
- 31 January - Hospital Marks Healthy Heart Month
- 22 November - Bermuda Hospitals Board Achieves Full Accreditation
- 7 November - Hospital Celebrates Advances in Diagnostic Imaging
- 27 October - Ethics Week 2011 Offers Food For Thought
- 12 August - Venetta Symonds To Be BHB CEO from 7 April 2012
- 6 April - Construction Begins
- 23 November - MindFrame PhotoVoice Exhibition Winners Announced
- 28 October - MWI Host Mental Health Forum for Community
- 28 October - Ethics Week Keeps it Confidential
- 13 October - Premier Visits Site of New Hospital Facility
- 10 September - BHB evaluates three bids
- 4 June - Parking Lot Takes Shape
- 4 May - KEMH Hosts Asthma Day Open House
- 26 March - Site preparation work begins
- 18 March - New Online Applications Process for BHB Jobs
- 23 December - Specifications for New Facilities Complete
- 23 December - BHB Releases Request for Proposal to Shortlisted Bidders
- 28 October - BHB Approves Shortlist of Qualified Bid Teams
- 27 October - New Chief of Nursing, Quality & Risk Appointed
- 14 October - Specifications Take Shape
- 10 September - New Director of Facilities Appointed
- 3 September - New Ambulance Fleet Unveiled
- 1 September - Pembroke Rotary Helps MWI Clients Snap into Action
- 25 August - Hospital Appoints Psychiatrists at MWI
- 29 July - Advisory Teams Evaluate KEMH Site
- 24 June - Robot Enhances Patient Care
- 11 June - World Blood Donor Day- June 14
- 8 May - Market Briefing Presentation
- 30 April - KEMH Hosts World Asthma Day Fair
- 16 March - Redevelopment Update
- 3 February - Presentation To Contractors
- 3 February - Presentation To Architects & Engineers
- 2 February - Hospital Opens New Pilot Patient Rooms
- 12 November - Johns Hopkins Phase II Report Released
- 20 October - Hospital Launches Infection Prevention Week
- 16 October - Mind Frame Art Exhibit Opens at City Hall
- 14 October - BHB Invests to Strengthen Nursing
- 29 July - Mother Support: Going for the Gold
- 27 March - Hospital Improves Laundry Service
- 23 January - Hospital Switchboard Undergoes 2-Hour Upgrade
- 20 December - New Director of Human Resources Appointed
- 27 November - Opening the Doors to Wound Care
- 11 October - Mind Frame Art Exhibit Opens at City Hall
- 6 September - Emergency Department Staff Receive Specialized Training
- 21 August - Dr Donald Thomas Appointed as Chief of Staff
- 15 February - Single Largest Donor Funds Improvements at Hospitals
- 1 February - Have You the Heart to Find Out Your Risk?
- 22 November - Day Hospital Provides Rehabilitative Services
- 17 October - Bermuda Hospitals Board Update on Estate Master Plan
- 16 October - Infection Control Week – It’s In Your Hands
- 9 October - Mental Health Awareness Week 2006
- 26 September - Deputy CEO Comments on Bermuda Hospitals Board Next Steps
- 21 September - Bermuda Hospitals Board Changes First Public Meeting Venue
- 20 September - Bermuda Hospitals Board Public Meetings
- 18 September - Safer and Faster X-ray Service Now Available to Patients
- 7 September - Community Open Houses to be Held at KEMH
- 10 July - BHB and BPSU Reach Three Year Agreement
- 21 February - Bermuda Hospitals Board Appoints Acting CEO
- 30 December - BHB Chairman Steps Down
- 12 September - Community Newsletter Available to Island Residents
- 6 September - Bermuda Hospitals Board Awarded Three-Year Accreditation
- 30 August - BHB Chairman Addresses Hamilton Rotary Club
- 7 April - Hospitals Launch New Web Site
- 1 November - November is Hospice Awareness Month in Bermuda
- 8 October - DEPRESSION
- 8 October - Schizophrenia
- 8 October - Substance Use
- 8 October - ANXIETY
- 7 October - Learning Disability
- 6 October - CHILD AND ADOLESCENT MENTAL HEALTH
- 6 October - CHILD AND ADOLESCENT DEPRESSION
- 5 October - Bermuda’s Mental Health Awareness Week
- 4 October - STIGMA AND MENTAL HEALTH